atoms to mol conversion|molecules to atoms calculator : Bacolod The online atoms to moles calculator finds the number of atoms to moles and moles to atoms of a substance. This conversion is important to find the molar concentration of the .
Resultado da 14 de fev. de 2024 · Drive on the heavy highway with multiple cars in multiple lane roads. Experience the accurate traffic elements with the traffic signals, headlights, turn signals, .
0 · multiply atoms and moles calculator
1 · molecules to atoms calculator
2 · molar mass to atoms calculator
3 · mol to atoms molecule
4 · mol to atoms calculator
5 · converting from atoms to moles
6 · convert mole to atom calculator
7 · More
8 · 1 mole to atoms
Horns They are replacement 90MM Gen Yam looking horns .
atoms to mol conversion*******This article explains how to convert between moles and atoms of different elements. It provides examples of converting the number of carbon, hydrogen, oxygen and sulfuric acid atoms into moles and vice versa. The article also highlights why it is important to know the chemical formula for a molecule in order to . See more
Typing subscripts and superscripts can be a hassle. Moles make life easier by converting back and forth between the number of particles and moles. See moreThere are 2 hydrogen atoms per water molecule; multiply given #molecules by 2 for answer; use conversion factors to find #hydrogen atoms in . See moreUsing unit conversion techniques, we can convert from the number of atoms to moles of atoms. See moreThe element carbon exists in two forms (graphite & diamond). 4.72 × 10^24 atoms of carbon is greater than Avogadro's number so it is more than 1 mole of C atoms rounded to three significant figures. See moreThe online atoms to moles calculator finds the number of atoms to moles and moles to atoms of a substance. This conversion is important to find the molar concentration of the .
Convert atoms to moles and moles to atoms using a simple calculator. Plus, learn the formula to convert atoms to moles.Do a quick conversion: 1 atoms = 1.660538863127E-24 moles using the online calculator for metric conversions. Check the chart for more details.molecules to atoms calculatorSimplify chemical calculations with our Atoms to Moles Calculator. Convert atoms to moles, moles to grams, and more. Accurate and user-friendly chemistry tool.
Need to convert between moles, molecular weight and mass? You can do it here with our mole calculator.
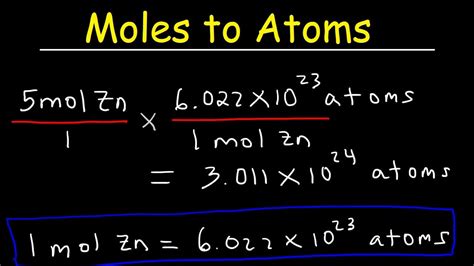
To convert from moles to atoms, multiply the molar amount by Avogadro's number. To convert from atoms to moles, divide the atom amount by Avogadro's number (or multiply .We can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. The study of the numerical .Do a quick conversion: 1 atoms = 1.660538863127E-24 moles using the online calculator for metric conversions. Check the chart for more details.This free online tool converts units of measurement for amount of substance - moles to atoms and vice versa.Conversion of moles to atoms. The mole is the unit of measurement for amount of substance in the International System of Units (SI). It is defined as exactly 6.02214076×10 23 particles, which may be molecules, atoms, ions or electrons, depending on the nature of the substance. The number 6.02214076×10 23 is known as the Avogadro’s number.A: Molar mass is found by adding up the atomic masses of all the atoms in a molecule. It is expressed in grams per mole (g/mol). Q: How many atoms in a mole? A: One mole contains Avogadro's number of atoms, which is approximately \(6.022 \times 10^{23}\) atoms. Q: How many atoms are equal to 1.5 moles of helium?The bridge between atoms and moles is Avogadro's number, 6.022×10 23. Avogadro's number is typically dimensionless, but when it defines the mole, it can be expressed as 6.022×10 23 elementary entities/mol. This form shows the role of Avogadro's number as a conversion factor between the number of entities and the number of moles. Example: Convert 2 moles of iron (Fe) to the number of atoms. Number of atoms = number of moles x Avogadro’s number Number of atoms = 2 moles x 6.02214076 × 10 23 atoms/mole ≈ 1.2044 × 10 24 atoms. Another way of calculating the number of atoms is through a two-step process: moles to grams and then grams to atoms. This . The first conversion factor converts from moles of particles to the number of particles. The second conversion factor reflects the number of atoms contained within each molecule. Figure 4.2.2 4.2. 2: Two water molecules contain 4 hydrogen atoms and 2 oxygen atoms. A mole of water molecules contains 2 moles of hydrogen atoms and 1 .We assume you are converting between atom and mole. You can view more details on each measurement unit: atom or mole The SI base unit for amount of substance is the mole. 1 atom is equal to 1.660538863127E-24 mole. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between atoms and .
Moles are a type of unit conversion used in chemistry to measure the amount of substances. One mole is equal to 6.022 x 10^23 atoms, molecules or other particles. By converting from moles to grams, liters, and other units, chemists can calculate the mass or volume of a given substance. This is especially useful when dealing with solutions and .
This chemistry video explains the conversion process of moles to atoms and how to convert the number of atoms to moles. This tutorial is useful for calculat.The molecular weight of sodium chloride, NaCl , is 58.44 g mol . How many moles of salt are in 13.8 g of sodium chloride? Express the answer using 3 significant figures. mol. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the .The simple unit conversion tool which helps you to convert atoms to moles or moles to atoms units. Skip to content. . Mole = Atom * 6.0221415E+23. Atom = Mole / 6.0221415E+23. where, Atom = Number of atoms; 1 Mole = 6.0221415E+23 Atom; 286. 3 4 votes. Рейтинг статьи .
6.02 x 10 23 is how many atoms are in a mole. The process of how calculating atoms from moles uses a conversion factor. To convert from moles to atoms (or other particles, such as molecules) in a . 1 mol Al = 26.98 g Al. Prepare a concept map and use the proper conversion factor. Cancel units and calculate. 3.987 molAl × 26.98gAl 1 molAl = 107.6gAl 3.987 mol Al × 26.98 g Al 1 mol Al = 107.6 g Al. Think about your result. The calculated value makes sense because it is almost four times times the mass for 1 mole of aluminum.
So to convert atom to mole just multiply atom value with 1.66053886313E-24. Let's see an example to convert 12 atoms to mol. As we are aware that 1 atom = 1.66053886313E-24 mole. Therefore 12 atom equals 12 x 1.66053886313E-24 mole which comes out to be 1.992646635756E-23 mole.We assume you are converting between atom and mole. You can view more details on each measurement unit: atoms or mol The SI base unit for amount of substance is the mole. 1 atoms is equal to 1.660538863127E-24 mole. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between atoms and . 6.02 x 10 23 is how many atoms are in a mole. The process of how calculating atoms from moles uses a conversion factor. To convert from moles to atoms (or other particles, such as molecules) in a . 1 mol Al = 26.98 g Al. Prepare a concept map and use the proper conversion factor. Cancel units and calculate. 3.987 molAl × 26.98gAl 1 molAl = 107.6gAl 3.987 mol Al × 26.98 g Al 1 mol Al = 107.6 g Al. Think about your result. The calculated value makes sense because it is almost four times times the mass for 1 mole of aluminum.Use this atoms to mol converter to convert amount-of-substance values from atoms to moles where 1 atom is equal to 1.66053886313E-24 moles. Enter value to find how many moles are in N atoms. See conversion chart and formula for reference.

We assume you are converting between atom and mole. You can view more details on each measurement unit: atoms or mol The SI base unit for amount of substance is the mole. 1 atoms is equal to 1.660538863127E-24 mole. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between atoms and .atoms to mol conversion To calculate this result: Calculate the molar mass of water, which is two hydrogen atoms' and one oxygen atom's molar masses combined: (2 × 1.008 g/mol) + 15.999 g/mol = 18.015 g/mol. Divide the mass of your sample by the molar mass: 100 g / 18.015 g/mol = 5.551 mol. This is the number of moles in 100 g of water.atoms to mol conversion molecules to atoms calculator To calculate this result: Calculate the molar mass of water, which is two hydrogen atoms' and one oxygen atom's molar masses combined: (2 × 1.008 g/mol) + 15.999 g/mol = 18.015 g/mol. Divide the mass of your sample by the molar mass: 100 g / 18.015 g/mol = 5.551 mol. This is the number of moles in 100 g of water.So, to find the number of hydrogen atoms in a mole of water molecules, the problem can be solved using conversion factors: 1mol H2O × 6.02 ×1023 moleculesH2O 1 molH2O × 2atoms H 1 moleculeH2O = 1.20 ×1024 atomsH 1 mol H 2 O × 6.02 × 10 23 molecules H 2 O 1 mol H 2 O × 2 atoms H 1 molecule H 2 O = 1.20 × 10 24 atoms H.The molar mass of an element (or compound) is the mass in grams of 1 mole of that substance, a property expressed in units of grams per mole (g/mol) (see Figure 3.5). Figure 3.5 Each sample contains 6.022 × × 10 23 atoms —1.00 mol of atoms.More information from the unit converter. How many mol in 1 atoms? The answer is 1.660538863127E-24. We assume you are converting between mole and atom.You can view more details on each measurement unit: mol or atoms The SI base unit for amount of substance is the mole. 1 mole is equal to 6.0221415E+23 atoms. Note that rounding .The first conversion factor converts from moles of particles to the number of particles. The second conversion factor reflects the number of atoms contained within each molecule. Figure 4.2.2 4.2. 2: Two water molecules contain 4 hydrogen atoms and 2 oxygen atoms. A mole of water molecules contains 2 moles of hydrogen atoms and 1 mole of oxygen .The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is "mol." Definition: Atom. This site uses an exact value of 6.0221415 x 10 23 for Avogadro's number. This is the number of atoms in 1 mole of a chemical element. Metric conversions and more6.5: Mole Calculations is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The molar mass of a substance is the sum of the average molar masses of the atoms that compose the substance. The molar mass of a substance can be used as a conversion factor between moles of the ..Conversions Between Moles and Atoms. Unit conversion techniques. All Modalities. Add to Library. Details.
Resultado da Transforme seu dispositivo em uma TV com o PlayTV GEH atualizado! Assista a jogos de futebol ao vivo e muito mais. Baixe agora e tenha a TV .
atoms to mol conversion|molecules to atoms calculator